Lasers in dentistry have been explored since the early 1960s. At its infancy, the practitioners of the field used Ruby lasers. They used the pulses emitted from such lasers to ablate the tooth enamel to treat defects and stains. However, safety concerns were raised about the spread of heat damage to the tooth interior. It was not until the advent of CO2 and solid state lasers that practitioners were able to experiment and find the right laser most suitable for such applications. Currently, lasers in dentistry are used to treat both the surface hard dental tissue on the outside and the soft dental tissue on the inside. In this article, we focus on the use of lasers in removing the photosensitive molecules from the surface of the tooth for whitening – a process dubbed “photobleaching”.
Photobleaching can be triggered by lasers to destroy the color centers in the pigmented compounds. This is mainly for aesthetic purposes. Modern solid state Er:YAG and diode lasers can be used for this purpose to prevent the shortcomings of the previously used CO2 lasers that caused excessive heating and collateral damage.
In this article, we will discuss the process of photobleaching induced by lasers, the methods used to bring this about, interaction of common lasers with dental tissue, and some practical factors to consider in usage.
Photobleaching
Photobleaching is the removal of the color centers by using light in certain compounds called chromophores. Chromophores are special kind of compounds that have a region in the molecule that is responsible for emitting light in the visible region. For example, the image below shows the color center in a beta-carotene molecule. The color center is the long red conjugated double bond structure.
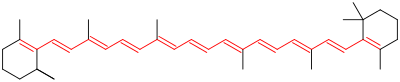
The red region indicates the active color center in this chromophore (Beta-carotene). Courtesy of Wiki.
Color centers, such as the one shown above, can be destroyed as the alternating double bonds are removed by some oxidizing agent. Gels such as hydrogen peroxide (H2O2) are often used as bleaching agents for this purpose. When light is incident on H2O2, the compound dissociates and creates a highly reactive oxygen – free radical. The free radical reduces the double bonds to single bonds and alcohols, which then can be removed. When the color center is reduced, the compound is said to be bleached.
Photobleaching Methods in Dentistry
In dental applications, photobleaching can occur via two distinct routes. In the first, the incoming laser energy can induce thermal excitation in hydrogen peroxide to cause dissociation, while in the second, it can directly cause the compound to dissociate. Thermal methods are currently preferred to maintain a controlled effect of free radicals.
The main characteristics that are desired in photobleaching lasers are selective activation, and ability to cause no damage to dental tissue during the treatment. Initial designs used plasma arc lamps and CO2 lasers with no gel for selective activation. This posed danger for dental tissue damage. To control this effect, gels such as hydrogen peroxide were used with laser wavelengths that do not cause strong absorption in the dental tissue. Er:YAG and diode lasers dominate for these purposes as they cause little to no dental tissue damage.
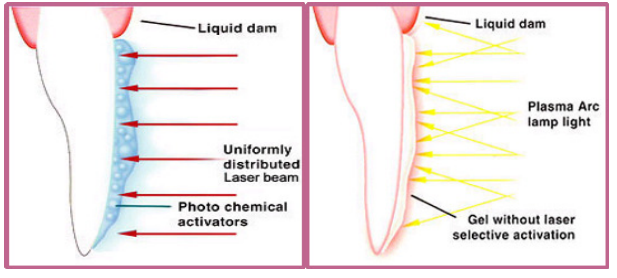
Left: Using gel for selective activation. Right: No gel was used in earlier designs.
Er:YAG Lasers for Photobleaching
Er:YAG lasers operating at 2940 nm are a good candidate for photobleaching. In particular, the energy of every such laser pulse does not exceed the ablation threshold of dental tissue at 3.5 J/cm2, which makes them a safe alternative. Moreover, the dental tissue does not absorb this wavelength strongly, while the wavelength is tuned for the gel’s thermal vibrational excitations instead. Using thermal photobleaching makes sure that the heat and free radicals are contained within the layer to be removed and minimal free-radicals reach the enamel.
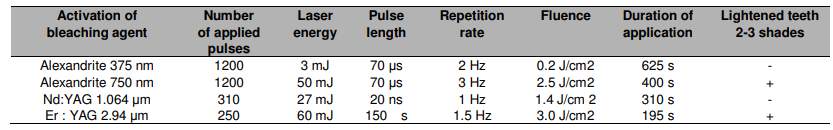
Comparison of some solid state laser characteristics for use in photobleaching. Courtesy of Medical Sciences.
Diode Lasers for Photobleaching
Diode lasers tuned from visible to NIR can also be used for photobleaching. They offer some advantages over solid-state lasers as they are small and have a compact setup. However, changing the wavelength choice increases the amount of heat absorbed by the dental tissue and care must be taken to make sure energies do not exceed the tooth enamel’s ablation threshold. On the plus side, diode lasers can be used for soft dental tissue surgery. These lasers are relatively poorly absorbed by tooth structure so that soft tissue surgery can be safely performed in close proximity to enamel dentin and cementum.
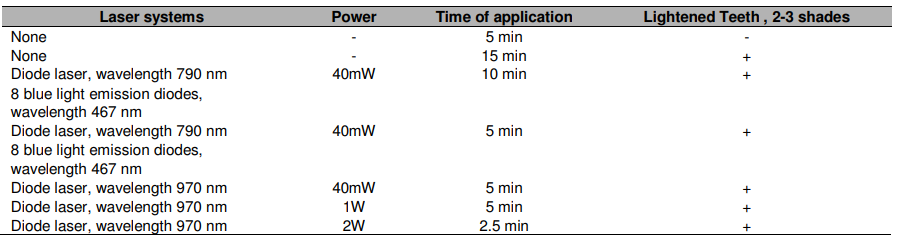
Common diode laser wavelengths and powers used for teeth whitening. Courtesy of Medical Sciences.
Conclusions
The image below shows the IR image of Er:YAG and diode based heating methods. Er:YAG only heats the hydrogen peroxide gel with little spread of heat to the enamel. In contrast, diode lasers cause the spread to the whole dental tissue including the gel on the surface.
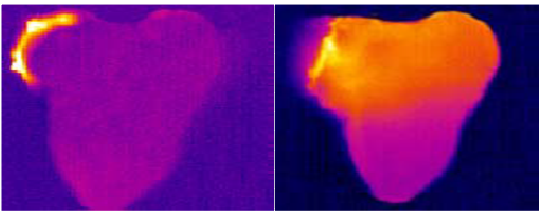
Left: Er:YAG for heating. Right: Diode lasers for heating. Diode lasers spread heat to the whole dental tissue.
Some practical matters to consider for the bleaching process is the time taken for the bleaching process, careful removal of byproducts, and the cost of operation. Costlier methods use specialized gels to attack more complex choromophores. Moreover, the whitening process itself requires a few visits to get a more satisfactory result.
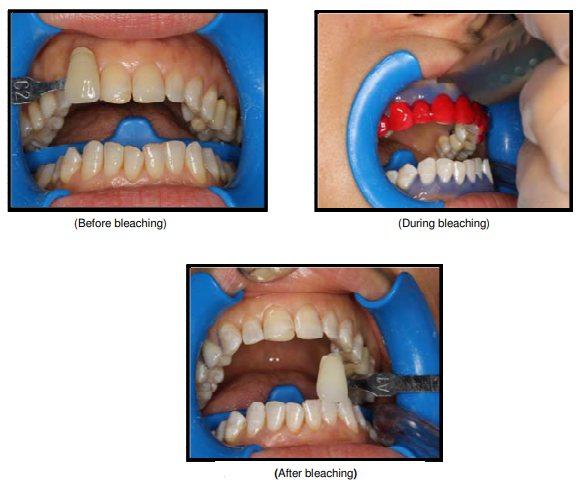
Photochemical bleaching process. The whitened teeth is shown at the bottom. Courtesy of Medical Sciences.
Er:YAG lasers have been useful for other dental applications as well because of their low penetration to the inner dental tissue. For example, they can be used to vaporize the enamel when used in pulsed mode. However, diode lasers are slowly starting to become more attractive for their compact size. The low power defects of diode lasers are being replaced by newer innovative pulsed diode lasers with higher energies.
To know more about the applications of Er:YAG in dentistry check out the following review article. Also check out FindLight’s vast collection of pulsed solid state and diode lasers to use in your setups.
