Non-linear techniques in photonics have opened up new avenues for sensing and manipulating materials. In the 1960s, non-linear techniques were used to discover multiphoton absorption processes in lanthanide doped nanoparticles. Moreover, lanthanides, such as Erbium, have already been used to make Erbium doped fiber amplifiers. In turn, these amplifiers have been used for long distance fiber-optic communications. Over the past few years, scientists have been exploring the ability of lanthanide nanoparticles to control biological reactions for medical applications.
A peculiar characteristics of lanthanides is that their excited states have really long lifetimes. It turns out, this property is very useful for fluorescence imaging and multiphoton absorption. The longer lifetimes give clearer images in fluorescence imaging, and stronger probabilities for multiphoton absorption. Apart from their use in imaging applications, they can also be used to detect biomolecules. By modifying the surface of the lanthanide nanoparticle, specific biomolecules can be absorbed onto the surface and be easily detected.
In this blog post, we will discuss the properties and synthesis of lanthanide nanoparticles, with their applications in photodynamic therapy and solar cells.
Properties of Lanthanide Nanoparticles
Lanthanide elements contain 4f orbitals that are shielded from the surrounding atoms by 5s and 5p orbitals. This causes the 4f energy levels in the lanthanides to be stable and protected from the surrounding atoms, giving rise to their longer excited state lifetimes and narrow linewidths in the emission spectrum. The plot below shows the emission spectrum of various lanthanides.
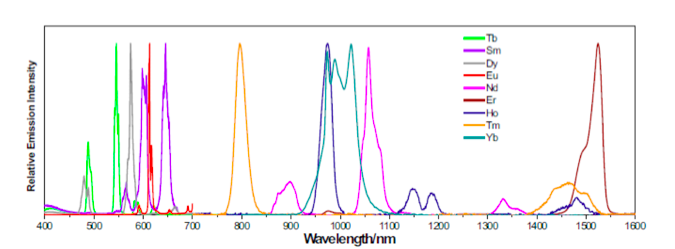
Emission spectrum of lanthanides. Courtesy of University of Pittsburgh
The emission spectrum of lanthanides show very little variations even when they are embedded in a crystal lattice environment. Elements such as Tb, Dy, Sm, Eu and Nd are seen to emit light in the visible spectrum.
Lanthanide Nanoparticles in Solutions
Nanoparticles are synthesized in a solution by precipitation techniques to control their size and composition. Hydro/solve thermal methods which involve high pressure and temperature, give good control over the shape of the particles, but take longer times to synthesize. Thermal decomposition methods give more control over the size and composition by controlling the temperature and solvent. Desired lanthanides are introduced in the solvent for a specific composition ratio. Nanoparticles nucleate and grow by Ostwald ripening in the above methods. Usually, additional materials are added to coat the surface of the nanoparticle to make it biocompatible and functional for detecting specific molecules.
Photon Upconversion
Multi-photon absorption is useful for converting low frequency infrared light to higher frequency visible light. There are many different mechanisms for multiphoton absorption, but the most efficient mechanism is energy transfer upconversion (ETU). A sensitizer atom and an emitter atom are required for this process, as shown in the image below. The emitter is the lanthanide nanoparticle, and the sensitizer is an atom with a comparatively smaller excited state lifetime.
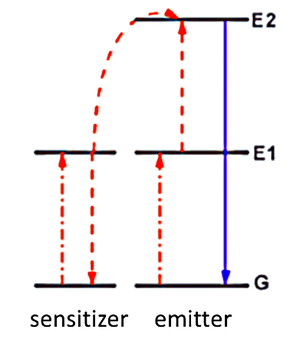
Energy Transfer Upconversion (ETU) using sensitizers and emitters. Courtesy Stanford University
The emitter is the main element that will emit the final high energy photon. The initial photon causes the emitter to transition from ground state G to the excited state E1. Meanwhile, a photon is absorbed by the sensitizer with the photon’s energy being comparable to the energy gap between E1 and G energy levels. Finally, resonant energy transfer happens, with the sensitizer losing its photon and causing the emitter to absorb the photon to move from E1 to E2. This is a favorable process, as the emitter is a lanthanide with a longer lifetime.
Energy transfer upconversion (ETU), while being the most efficient, is not the only absorption process that takes place. The nanoparticles must be carefully designed to make sure that other processes are not dominant.
Key Applications
Lanthanide nanoparticles find their uses by inducing reactions in nearby molecules. In this article we address two such applications relating to cancer treatment and improvement of solar cell efficiencies.
1. Uses in Photodynamic Therapy
Targeted therapy is a more effective form of cancer treatment than chemotherapy. Photodynamic therapy (PDT) is one such method. Cancer cells are selectively populated with light sensitive materials. When external light is targeted at the region, reactions that induce cell death are triggered. This does not kill the nearby non-cancerous cells while selectively targeting the cancerous cells.
A recent article showed that lanthanide nanoparticles when functionalized with zinc beta-carboxythalocyanine (Zn-COOH) at the surface, was a good candidate structure for PDT. The nanoparticle can be activated by near infrared light, when it is inside a tumor cell. The IR light is used to trigger multiphoton absorption in the lanthanide element. The Yb/Er lanthanide ion complex is used to emit visible light from IR light by energy transfer upconversion (ETU). The emitted photon is absorbed by the nearby Zn-COOH causing it to breakdown, with oxygen radicals being released in the process. In their turn, these oxygen radicals induce cancerous cell death. The image below illustrates the outlined process.
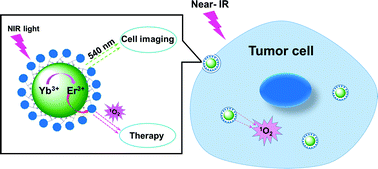
Cell death induced by lanthanide nanoparticles . Courtesy Nanoscale
2. Uses in Solar Cells
Solar energy in the IR region is not fully absorbed by a solar cell. Moreover energy in the UV spectrum is not fully utilized for conversion to electrical energy. The image below shows the various regions in the EM spectrum that can be converted to region in the absorption spectrum of solar cells. Lanthanide nanoparticles can be used to aid the conversion of IR light into light that can be absorbed by a solar cell.
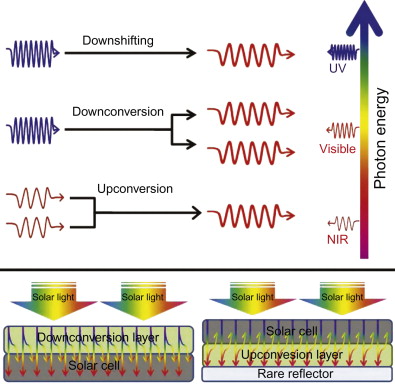
Upconversion from IR region to visible region is used to enhance solar cell absorption. Courtesy of ScienceDirect
Upconversion is a process where many low energy photons can be absorbed and converted to a single high energy photon. Multiphoton absorption is a type of upconversion. Since lanthanide nanoparticles can be used for multiphoton absorption, they can be used to convert light from the IR region into the visible region for solar cells. By adding a layer of lanthanide nanoparticles optimized for upcoversion, photons far in the IR region are converted into photons in the absorption spectrum of the solar cell. Thus more energy from the incident light can be converted into electrical energy, increasing energy efficiency.
These are only some of the key applications of lanthanide nanoparticles utilizing multiphoton processes. Did you know, FindLight’s vast collection of products from leading vendors provide setups for fluorescence spectroscopy, diode laser solutions, and photodetectors.
