Light Sheet Microscopy (LSM), a.k.a. single plane illumination microscopy (SPIM), is a technique that allows for high resolution imaging of very small structures. This makes it one of the most popular techniques in the biomed market today. Particularly, it is used to image cells and other biological systems. Among the reasons why it is so popular is because it fills a need not met by its counterparts. Conventional microscopes utilize a common path and axis for both imaging and illumination. To clarify, the whole sample needs to be illuminated and the results include out of focus areas. Thus, a lot of noise is generated and the results are hard to read. The signal may be improved through higher intensities of light. However, the sample may receive worse phototoxic effects and the results will not be accurate.
Another alternative is the confocal microscope and spinning disk confocal microscope. These utilize pinholes where light in the focal plane is detected. Unlike other methods, out of focus areas are not detected. Indeed, this provides a more clear signal, but out of focus areas are still being somewhat illuminated. Again, the system in question will start to decay. Hence, we would not know if the results were from a healthy or decaying state.
With this in mind, we need a microscope that allows for long-term observation, minimal photodamage, and clear data. Light Sheet Microscopy fits all of the above and more. It was popularized in 2004 as SPIM. It also stems from the ultramicroscope introduced by Zsigmondy and Siedentopf in 1903. The term Light Sheet Microscopy encases several variations and techniques. Among these are: Light Sheet Fluorescence Microscopy (LSFM)/ L-SPIM, diSPIM and mSPIM. In this article we will discuss one of the variations of LSM in the biomedical market and the principles behind the technology.
Figure 1
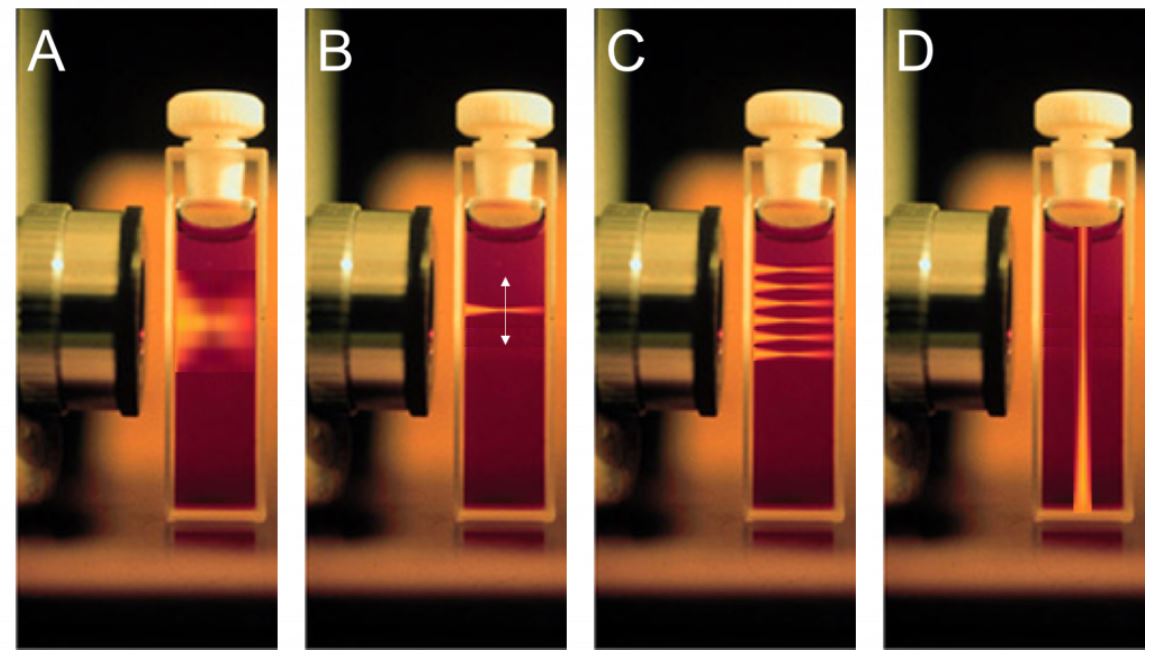
The beam distribution of four different microscopy techniques. A) Widefield microscopy. The beam illuminates the entire sample above and below the plane of focus. B) A higher powered confocal laser beam. Illuminates the sample at a specific point. C) The confocal laser focus point is small so it requires scanning across the sample. D) Theoretical layout of a light sheet system. The excitation beam is placed perpendicular to the imaging axis.
Courtesy of Photometrics.
Principle of Light Sheet Microscopy
The setup of Light Sheet Microscopy is different from other conventional systems. This is because the illumination beam is placed perpendicular to the imaging instruments. When the microscope is turned on, the imaging plane then coincides with the focal plane. Thus, the instrument captures only one 2D plane of the sample at a time. Hence, there is very little background noise. Additionally, a cylindrical lens is placed in front of the laser beam which shapes the beam into an hourglass shape. As mentioned before, we want the sample to be exposed to as little light as possible. For this reason, the sample can be placed at the thin waist of this hourglass beam. Now, the light from the sample is directed up towards the detector/camera through the other arm of the setup. This is by means of another objective and a lens, similar to conventional setups.
Benefits of LSM
Other than minimizing sample exposure, this setup has many benefits. The sample can be imaged quickly and in small sections. Thus, fast moving systems can be recorded in detail with great clarity. Furthermore, imaging in sections eliminates the production of excess noise. Another huge benefit to this system is that the light sheet can be rotated around the sample in 3D to get multiple views in multiple planes. Converse to LSM, conventional systems require the sample to be manually rotated in 3D to capture different angles. Additionally, it should be noted that a stitched up 3D image is far superior in resolution compared to whole 3-D snapshots. Moreover, composite images can be created within seconds to minutes. On the other hand, it takes minutes to hours for other microscopes. Clearly, Light Sheet Microscopy setups allow for much more dynamic and efficient imaging.
Figure 2
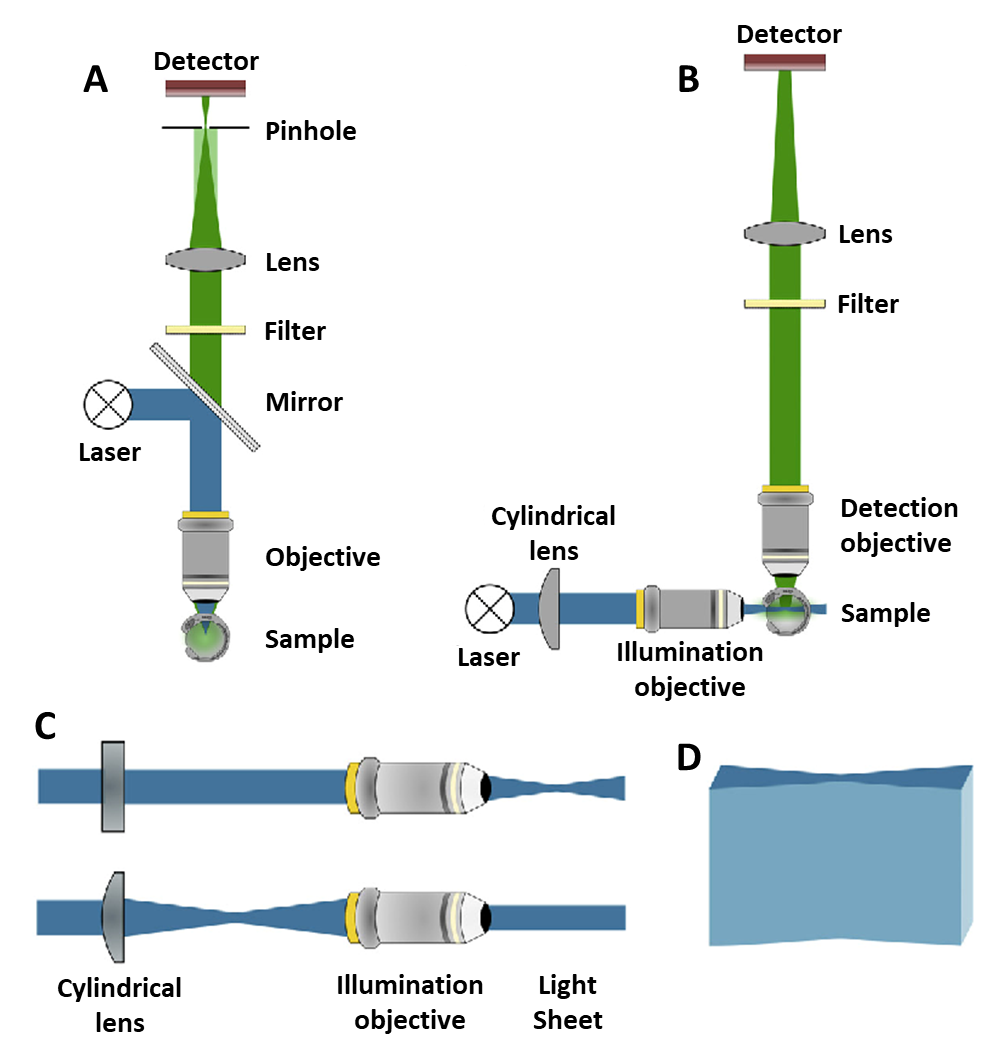
The light path in light sheet vs confocal microscopy. A) A confocal light path. The blue excitation laser illuminates the sample through the same objective as the green detection pathway. Light passes through a pinhole. B) A light sheet light path. The blue excitation light sheet is perpendicular to the green detection pathway. Two objectives are needed but there is far less out-of-focus light. C) The cylindrical lens used to make the light sheet. Seen from above (top) and the side (bottom).
Courtesy of Photometrics.
Light Sheet Microscopy Applications- Prostate Cancer
Prostate cancer is one of the leading causes of cancer deaths for males worldwide. The current main form of treatment is a prostatectomy, which completely removes the whole prostate. After removal, histopathology exams are required to discover the location of tumor nodules. However, these exams are not the most efficient or accurate because of the size of the organ and the exam method. During the exam, the prostate is sliced into sections and put onto microscope slides for examination. To further clarify, these processes include chemical fixation, tissue embedding, staining, and microscopic evaluation. The histopathology process is very laborious. In fact, most of these samples do not even include tumorous tissue. Thus, 90% of labs do not evaluate the whole organ. However, this makes the exam very inaccurate and has been shown to produce less healthy patient outcomes.
Light Sheet Microscopy is one method that can eliminate this blind testing. Specifically, we will discuss a method that images prostate tissues utilizing a wide surface area and open-top light sheet microscopes. LSM methods do not require slides and consume less resources to test per slice. Further, intact tissues can be viewed in 2D and 3D at equally high resolution as conventional pathology methods. In a study done with Nature Biomed Engineering, 25 prostate slices (3.1 x 3.5 x 0.4 cm) were brought. They were stained with fluorescent dye and rinsed in preparation. The slices were then placed into a open-top light sheet microscope (at image height 320 μm, imaging speed, v = 50 sec/cm2). Average imaging times were less than 10 minutes per slice.
Figure 3
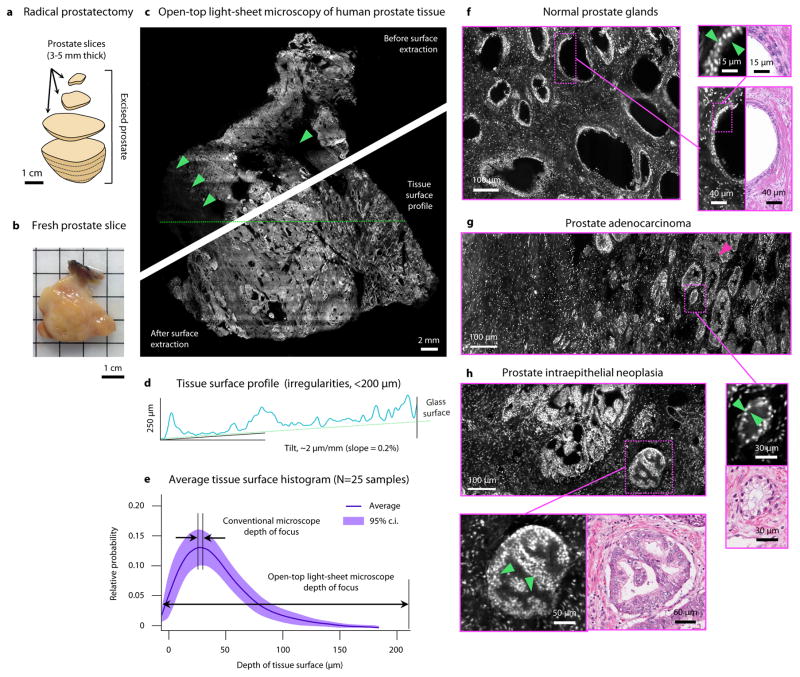
Images of preparation stages, results, and analyses of human prostate tissues from patients with prostate cancer.
Courtesy of Nat Biomed Eng.
A 3D composite image was formed using a digital surface-extraction algorithm. Significantly, the prostate tissue surface irregularities were less than 200 μm. This is a good sign of image quality because the LSM’s depth of focus and vertical field of view is greater than 200 μm. This study shows that Light Sheet Microscopy methods enables an accurate triage of normal to abnormal prostate slices. Additionally, the LSM processes allow for a more rapid and accurate diagnoses of tumorous tissues compared to conventional methods that would take several hours more. Since LSM methods also provide 3D images, estimates of tumor location, grade, and size can be made by doctors.
Video 1.
A video of an entire prostate surface, showing the ability to zoom in and out.
Conclusion
Light Sheet Microscopy allows biologists and researchers to image both large and small specimens. The subjects can be viewed under longer and healthier conditions than other conventional microscopy methods. This is due to how the technology is setup. It illuminates only one plane of the sample at a time for short periods. Through this method, the sample is exposed to 20-100 times less light than the competition, improving sample lifetime. Additionally, the specimen can be rotated easily and image any plane with multiple angles. Hence, we can produce a high-resolution and 3-D image. Furthermore, this method is perfect for live specimens such as animals and cells that are photosensitive and need longer monitoring periods. LSM is also a very active area of R&D, with new techniques being evolved for specific uses in labs and commercial usage.
This post is sponsored by DataRay, the leading manufacturer of laser beam profiling solutions.
