Cardiac tissue imaging: cardiac diseases are a prevalent problem today. One medical challenge is getting high quality images of the nutrient and fat rich cardiac tissue. Courtesy of Cleveland Clinic.
Researchers at the Cardiovascular Surgery Department at the Chinese Academy of Medical Sciences and Peking Union Medical College have developed a new method for intact cardiac tissue imaging. This method, named Scheme Update on tissue Transparency (SUT), was presented in a paper earlier this year. Using this method for cardiac tissue imaging allows researchers to view cardiac diseases more clearly. Researchers were able to combine SUT with Electrophoretic Antibody Labeling (EAL) and other imaging techniques to gain a clear image of molecules within the cardiac tissue. Compared to other methods, this novel procedure causes minimal damage to the cells and requires less time.
What cardiac tissue imaging techniques are currently being used?
Obtaining transparent cardiac tissue has been a goal of scientists for a century now. In the early 1900s, it was beneficial as it allowed for anatomical studies. Today, transparent tissue prevents scattering and absorbance of light during imaging. Removing lipids and heme in the heart is essential for cardiac tissue imaging and for the analysis of molecular changes in the heart due to diseases.
Currently, non-cardiac-specific chemical combinations used for whole body imaging are the standard for clearing cardiac tissue. These chemical combinations include Scale, SeeDB, CUBIC, PACT, BABB, and CLARITY. The major disadvantage of these chemical combinations is their slow speed. CUBIC is the most widely used. However, it causes between 24% and 41% protein loss. Another widely used chemical combination is PACT-PARS. Although it does not cause major protein loss, this method is not as effective as CUBIC in clearing the tissue. Additionally, it causes tissue swelling.

This image displays cross sections of cardiac tissue. These tissues were exposed to multiple cardiac tissue clearing agents for five days. Courtesy of Biomedical Optics Express.
What are the benefits of Scheme Update on tissue Transparency?
Scheme Update on tissue Transparency is a new chemical combination. It functions as a cardiac tissue-clearing agent. The chemicals in this solution are Urea, TritonX-100, and SDS dissolved in PBS. First, PBS was used to clean the cardiac tissues. Then, the samples incubated in the SUT solution for four to six days for the mouse and rat hearts. The pig heart required 29 days of SUT exposure. This is much faster than other methods, which require a week or longer for the smaller mouse and rat hearts.
The SUT solution minimized protein loss and antibody loss. Images were made of the treated tissue after they were incubated with antibodies. Based on data from light-sheet microscopy and single-photon confocal microscopy, it was evident that these small molecules were retained within the tissue. The images were clear and there was little problem with light absorption or scattering. One noted problem with the technique was that imaging had to be done soon after incubation. Freezing samples or repeated exposure to SUT after staining would lead to a loss of fluorescence.
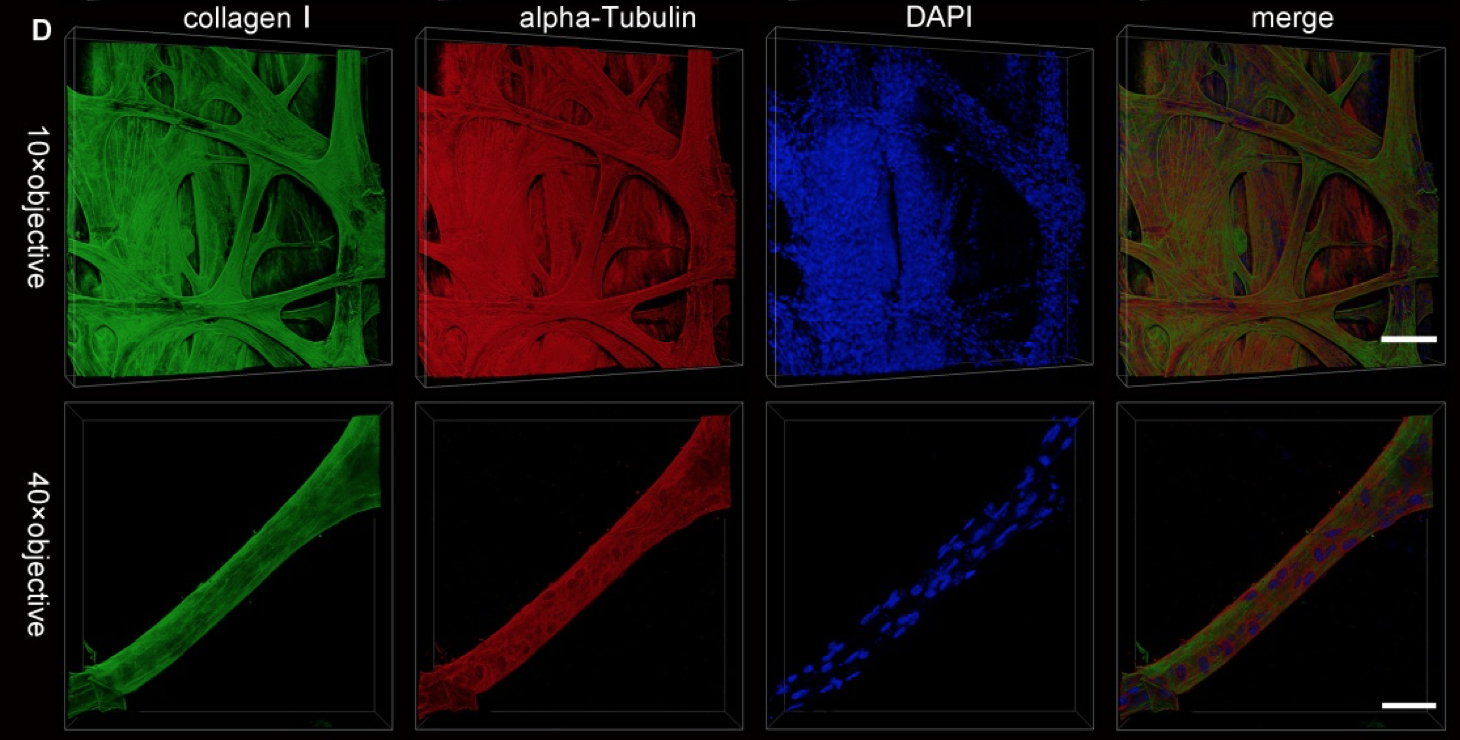
Single-Photon confocal microscopy was used to make these images following antibody staining of SUT-treated cardiac tissue. Tissues were incubated with the antibodies after 5 days of the SUT treatment. Courtesy of Biomedical Optics Express.
The effect of SUT on biomedical imaging
As stated earlier, cardiac tissue imaging is a challenge for researchers. Lipids between tissue layers scatter light. In addition, chromophores like heme absorb light. Therefore, whole-layer light transmittance is very difficult. These challenges must be addressed before clear and high definition images can be taken. This study has proved that it is possible to eliminate these challenges while still maintaining a relatively undamaged tissue.
In the future, this method can be optimized. Doing so will open doors for all kinds of biomedical imaging. Clearing tissues of components which hinder light during imaging techniques such as confocal microscopy is important progress. Better cardiac imaging will provide greater insight into the progression of diseases such as myocardial infarction and hypertrophic cardiomyopathy. Furthermore, this tissue clearing technique has shown preliminary success with brain tissue. It could also be used as a non-specific technique for clearing any tissue within the body.
Further improvements to SUT could include decreasing tissue damage even more. The team of researchers has committed themselves to focusing on the combination of SUT with antibody and molecular staining. In conclusion, with further developments, the SUT technique has high potential as a vital component of cardiac imaging.
To read more on this helpful technique for tissue imaging, the paper can be found here.
