Introduction to Dye-Sensitized Solar Cells
Dye-sensitized solar cells have the potential to replace traditional and costly crystalline silicon solar cells. The sun emits about 3.86 x 1026 joules every second. Approximately 1.74 x 1017 watts actually reaches the earth. If even a small fraction of this power output could be harvested, any existing or impending energy crises would be solved. The potential to harvest the sun’s energy lies in solar cell technology. A solar cell is an electronic device which directly converts sunlight into electricity. The specific type of solar cells this paper discusses is known as dye-sensitized solar cells. This type of solar cell requires a sensitizer to absorb sunlight. This sensitizer is usually a molecular dye. To reiterate, dye-sensitized solar cells serve as an environmentally and economically friendly substitute for traditional crystalline silicon or ruthenium solar cells because of their use of organic substances and relatively inexpensive fabrication.
Due to their relatively simple fabrication, dye-sensitized solar cells have been studied in recent years; however, little improvement has been made with regards to their energy efficiency. For example, ruthenium complexes are often used as dye in dye-sensitized solar cells; unfortunately, this type of sensitizer is quite expensive to manufacture. As a result, finding an alternative dye is the key in optimizing the performance of dye-sensitized solar cells.
Characteristics of a Dye-Sensitized Solar Cell
Anatomy of a dye-sensitized solar cell
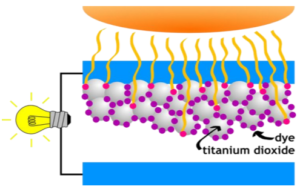
Anatomy of a DSSC. Sunlight hits the negatively charged electrode, also known as the anode. Attached to the anode are titanium dioxide molecules (pictured as white spheres) which are then dyed or stained by quantum dots (displayed as purple spheres). The surface on the bottom serves as the positively charged electrode, also known as the cathode. Courtesy of Brigham Young University.
One of the major components in a dye-sensitized solar cell is the porous layer of titanium dioxide. This layer is coated with a molecular dye that absorbs sunlight (see figure above). The dye works as an electron acceptor and an electronic conductor. The choice of semiconducting atoms used in the growth of the nanoparticles/quantum dots can determine the range of wavelengths of sunlight that can be absorbed. To complete the fabrication process of a dye-sensitized solar cell, electrolyte must be placed between the anode and the cathode. The stability of any particular dye-sensitized solar cell is very dependent on the electrolyte.
Band Gap of a Semiconductor
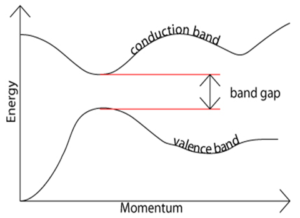
Band Gap Energy. When the energy of an electron is plotted as a function of momentum, the energy difference between the conduction band and the valence band represents the band gap. This particular graph depicts direct band gap since the momentum stays constant as the electron is raised to the conduction band. Courtesy of University of Cambridge.
A semiconductor is a solid substance that has conductivity between that of an insulator and that of most metals. The main characteristic property of a semiconductor is its band gap. The band gap of a semiconductor is the minimum energy required to excite a ground-state electron to a free state where it can participate in conduction (see figure above). According to the Brus equation,
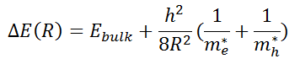 , band gap energy is inversely proportional to the size of the nanoparticles. The relationship can be described by the equation above where ΔE represents the band gap energy of the quantum dot, Ebulk represents the band gap energy of the bulk semiconductor, R is the radius of the quantum dot, me* is the effective mass of the excited electron, mh* is the effective mass of the excited hole, and h is Plank’s constant.
, band gap energy is inversely proportional to the size of the nanoparticles. The relationship can be described by the equation above where ΔE represents the band gap energy of the quantum dot, Ebulk represents the band gap energy of the bulk semiconductor, R is the radius of the quantum dot, me* is the effective mass of the excited electron, mh* is the effective mass of the excited hole, and h is Plank’s constant.
Therefore, by manipulating the size of the quantum dots, the band gap energy can be tuned accordingly. In reference to the figure above, when the energy of an electron is plotted as a function of momentum, the energy difference between the conduction band and the valence band represents the band gap. This particular graph depicts direct band gap since the momentum stays constant as the electron is raised to the conduction band. The band gap energy of our semiconducting quantum dots determines which wavelengths of sunlight can be absorbed by our solar cell.
Efficiency of a Solar Cell
This section will cover three different characterizations of solar cell efficiency: fill factor, traditional efficiency, and quantum efficiency. In regards to calculating these various efficiencies, experiments are commonly conducted using arc lamps with air mass filters. The post-filter intensities are usually set to about 100 mW·cm-2.
Fill Factor
The performance of a dye sensitized solar cell can be characterized in many ways. A fill factor is the maximum power output over the product of the open-circuit voltage and the short-circuit current. The fill factor is essentially a measure of the quality of any particular solar cell.
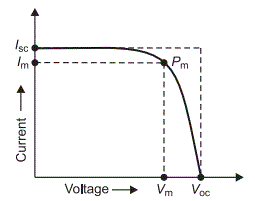
General I-V curve. Defined as the the maximum power output over the product of the open-circuit voltage and the short-circuit current. Courtesy of Electrical4U.
Traditional Efficiency (η)
Traditional efficiency is simply a ratio of the power output over the power input.
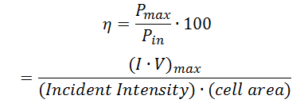
As shown above, the efficiency of the solar cell is calculated using the equation above where Pmax is the maximum electrical power output and Pin is the product of the irradiance light and the surface area of the solar cells.
Quantum Efficiency (IPCE)
In addition to calculating traditional efficiency, quantum efficiency can also be used to characterize a solar cell’s efficiency.
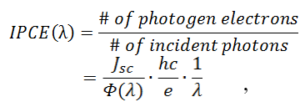
Quantum efficiency is also referred to as “incident photon-to-electron conversion efficiency” (IPCE). It is the ratio of the number of carriers collected by the solar cell to the number of photons of a given energy incident on the solar cell. Jsc is the current density produced under monochromatic illumination of the cell. Ф(λ) is the incident radiative flux that strikes the cell at a particular wavelength; h is Plank’s constant, c is the speed of light, e is the charge of an electron, and λ is wavelength. The quantum efficiency may be given either as a function of wavelength or as energy.
Common Binding Methods for Dye-sensitized Solar Cells
This section will cover three common binding methods for organic solar cells. As mentioned previously, light absorbing quantum dots are being binding to the titanium dioxide to act as a sensitizer for our solar cell.
Immersion Method
Perhaps the simplest and most widely used process, the immersion method, simply involves staining the titanium dioxide surface in a mixture containing quantum dots. The immersion time is entirely dependent upon the concentration and content of your quantum dot solution. Some research shows that dyeing the titanium dioxide fully may take up to 72 hours.
SILAR
An alternative binding method utilized is successive ionic layer adsorption and reaction (SILAR). The SILAR growth process involves deposition through alternate immersion in anionic and cationic solutions interspersed with rinsing in distilled water at room temperature.
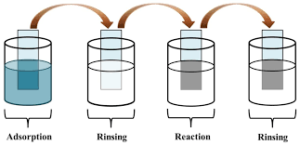
SILAR method of deposition. Involves deposition through alternate immersion of the treated substrate in anionic and cationic solutions interspersed with rinsing in distilled water at room temperature. Courtesy of Wikipedia.
Electrophoretic Deposition
Electrophoretic deposition is a technique that involves the movement of suspended charged particles due to an applied electric field. This electric field allows these charged particles to cast onto any shaped substrate.
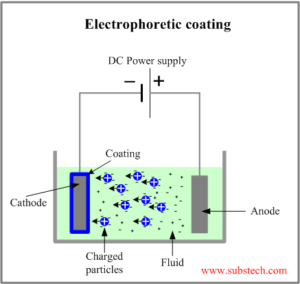
Electrophoretic deposition. involves the movement of suspended charged particles accelerated by an applied electric field. Courtesy of Substech.
The theoretical maximum efficiency of a single band gap dye-sensitized solar cell is 33.16%. This efficiency is significantly greater than the maximum efficiency of silicon based solar cells which is between 12% and 15%. If researchers could fabricate an organic solar cell using durable and versatile electrolyte at efficiencies near the theoretical maximum, dye-sensitized solar cells have the potential to not only replace silicon based solar cells, but also become one of the most relevant sources of clean energy generation.
