The mid-IR free-electron laser has been used in the medical sector for some time now. Laser usage in medicine has opened many doors and created countless avenues for surgery and forms of therapy. Recently, groups led by Dr. Kawasaki from the Tokyo University of Science in Japan and Dr. Nguyen from the Centre National de la Recherche Scientifique in France have found another use for free-electron lasers in medicine.
Background
Advances in medicine have made previously incurable diseases curable, resulting in the well-being of many. Sadly, there are still some diseases that remain not only painfully incurable, but also have no truly effective treatment options. One of these diseases is Alzheimer’s disease. Alzheimer’s is a neurogenerative disease with one of its trademarks being producing amyloid proteins that turn into amyloid fibrils. Amyloid fibrils are “abnormal fibrous, extracellular, proteinaceous deposits found in organs and tissues”. These fibrils aggregate in β-sheet structures where backbone hydrogen bonds are formed.
This change in structure makes the normally soluble proteins insoluble. These protein structures can then build up between neurons and alter cell function. Although the amount of amyloid proteins and severity of a patient’s Alzheimer’s has been found to be uncorrelated, minimizing secondary nucleation of these proteins is still desirable. Secondary nucleation is a process where new proteins are formed from the presence of parent proteins, so eliminating the parent proteins would limit the potential for more of these abnormal fibers from forming.
Previous Methods
Former attempts to get rid of these fibrils include introducing antibodies to target the so-called Aβ proteins. This method has been unsuccessful, as antibodies generally target a specific molecular process which can lead to induced toxicity. Other methods include using repeated scanning ultrasound. The ultrasound was shown to be able to dissociate the proteins in vitro in a mouse brain. However, the potential for using this method for humans is questionable. The hippocampus and medial temporal lobes are typically the most affected areas of the brain for those with Alzheimer’s. These regions are deep in the brain, which could make it difficult for the ultrasound to efficiently irradiate the areas.
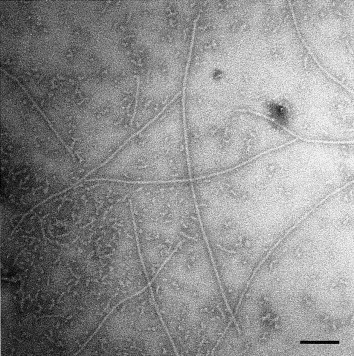
An electron micrograph of amyloid fibrils in water. Courtesy of Biochimica et Biophysica Acta
Free-Electron Laser Study Background
This leads to the method out of Tokyo University, published in June, where proteins were depolymerized using a tuned free-electron laser (FEL). The FEL, when coupled with microscopy methods, reveals information about the dissociation of the fibril. However, it has limited resolution that also limits understanding of the dissociation process. Therefore, they have coupled the free-electron laser with a laser-induced nonequilibrium molecular dynamics (NEMD) simulation method. NEMD provides all the information the group deemed necessary but is used with a more intense laser for shorter periods. Their experiment and study results were compared to confirm that the combination of the two techniques would yield useful information. The tests were conducted on a yeast protein that naturally forms amyloid fibrils. Their findings concluded that mid-infrared (mid-IR) FEL combined with an all-atom NEMD simulation causes amyloid fibril dissociation.
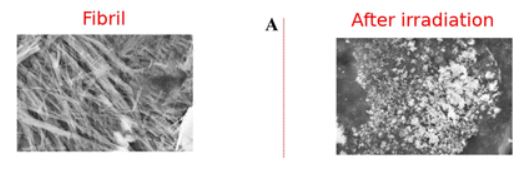
The image above shows a fibril (left) before and after (right) irradiation. Courtesy of the Journal of Physical Chemistry
Free-Electron Laser Experimental Methods and Results
To conduct the experiment, the peptide was dissolved in acetic acid, stored, and incubated. The solution then underwent irradiation using a mid-IR FEL oscillation system. The free-electron laser pulse was directed into a gold-coated mirror that reflected it through a BaF2 lens onto the prepared peptide sample. The team obtained IR spectra of the absorbance versus wavenumber for the amide I band of the fibril for both a mature-fibril and a pre-fibril. This is necessary, as the methodology “relies on the resonance between the laser frequency and the frequency of the amide I band of the fibril”. This analysis led the team to determine the resonance wavelength was 1631 cm-1 and the FEL was set accordingly.
To monitor the fiber formation and dissociation, they used Thioflavin T (ThT). The fluorescence intensity of the ThT fibril was recorded both at a non-specific wavelength and the previously determined resonance wavelength. With the non-specific wavelength, the ThT fluorescence signal did not differ greatly from that of the fibril, even at high power, indicating that the fibril structure had not changed. Conversely, the fluorescence signal after being irradiated at 1631 cm-1 was decreased by roughly 85%, indicating “full destabilization of the fibril”.
Simulation Methods and Results
The team then modeled the molecular dynamics for the fibril state. Out of the models created, 100 of them were then selected to for use to perform laser-induced (NEMD) simulations. Each of these structures had an IR spectrum calculated for them. The IR spectra were then averaged, leading to an amide I band at 1675cm-1 corresponding to the cross-β structure. Using the NEMD simulation, they found that the “most significant fibril deformation after one single laser pulse” occurred at this wavelength. The resonance wavelength in the simulation differed from that in experimentation since the simulation is considering molecular mechanics force fields and the experiment dealt with spectroscopic force fields.
To confirm the simulation data matched that from the experimentation, the amount of secondary structures was calculated for the sample after irradiation for the NEMD simulations and compared them to the FT-IR results. Overall, the calculations agreed with the experimental data. Cross-referencing with their previously conducted work, the group also determined that the dissociation was not due to thermal effects but “primarily due to the excitation of the amide I band vibration”.
This, along with the data from non-resonant wavelengths, supports that is the strong resonance between the laser and the amide’s vibrational band that causes the dissociation of the fibrils. The group sees the free-electron laser as a promising tool to treat Alzheimer’s disease. Their current work is to improve upon this study and be able to directly compare the experimental and simulation spectra.
This blog post was sponsored by Gentec EO - world leader in laser beam measurements.

These protein structures can then build up between neurons and alter cell function. Although the amount of amyloid proteins and severity of a patient’s Alzheimer’s has been found to be uncorrelated, minimizing secondary nucleation of these proteins is still desirable. Secondary nucleation is a process where new proteins are formed from the presence of parent proteins, so eliminating the parent proteins would limit the potential for more of these abnormal fibers from forming.