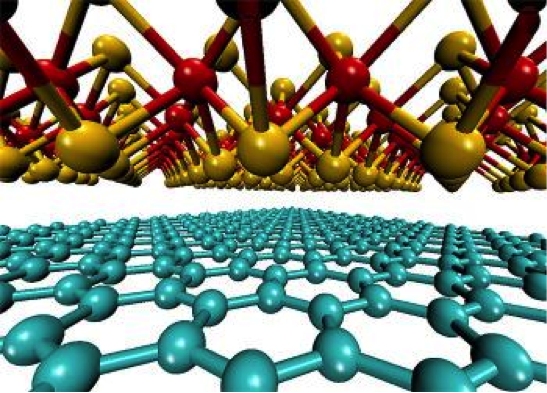
A research team from AMBER, Ireland, has discovered a new research method to produce large volumes of high-quality graphene for industrial use (Courtesy of Engineers Journal)
Graphene (a form of fullerene) is a nanomaterial that has been attracting significant interest as a potential game changer for various applications. Energy storage, flexible transparent displays and mechanical supports are only a few of the promising areas of such applications. Graphene is a honeycomb-patterned sheet of carbon atoms, with a thickness of one carbon atom. This makes it a two-dimensional material, which despite its thinness manages to be 200 times stronger than steel. It is also almost entirely transparent and an excellent conductor of heat and electricity.
In the past few decades, fullerenes have been a center of active research investigations thanks to their extraordinary physical, chemical, and mechanical properties. These properties makes them an outstanding material for future electronics, optics, and energy-harvesting applications. To date, a number of different types of solar cells have used graphene electrodes. This includes solid-state solar cells, electrochemical solar cells, quantum dot solar cells (QDSCs), and polymer solar cells.
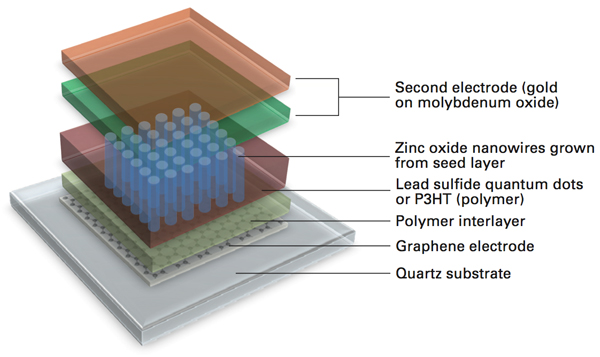
Solar cell with nanowires and graphene electrode (Courtesy of MIT Energy Institute)
So why is Graphene so Unique?
Graphene is an atomic-scale honeycomb lattice made of carbon atoms. Because of its two-dimensionality it does not lend itself to classic laws of three-dimensional world. Many of the physical/chemical properties exhibit a behavior that is unique to this two-dimensional construct of the material.
Graphene molecular mesh
In photovoltaic cells, they create an absorption window in a wide range of wavelengths (from UV to far IR region). Moreover, it exhibits a high charge transfer kinetics at the interface of electrochemical hybrid cells allows manufacturing of flexible constructs with robust architecture, while providing great heat dissipation.
In addition to fullerenes, quantum dots have also gained popularity in the photovoltaic community. For instance, the third generation photovoltaic devices now include semiconductor quantum dots (SQD). Similar to organic photovoltaic cells (OPVs), and die-sensitized solar cells (DSSC), SQDs offer an enhanced conversion ratios yielding shorter energy payback times.
Fullerenes come in handy also in DSSC type cells, which benefits from its unique properties that include rapid electron transport, extraordinary light transmittance, and widely tunable electrochemical properties. The photo anode of a DSSC functions as an electron “vehicle” to transport the injected electrons from the excited dye sensitizers to the outer circuit. In this point of view, graphene is a promising composite candidate to TiO2, which markedly facilitates the electron transfer from TiO2 to a current collector. The reason behind it is the band position of graphene which lies between the fluorine doped tin- oxide and titanium dioxide.
What does Future Hold for Fullerenes?
Researchers from MIT are working towards developing a new solar cell, made from graphene and molybdenum disulfide. They promise it to be thin, light and efficient (up to a 1,000 times more than silicon panels). Researchers from the same university also developed a flexible transparent graphene-based electrodes for polymer solar cells. It is arguably the most efficient electrode of its kind ever developed.
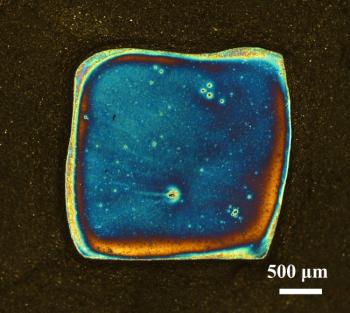
A 65-nm-thick titanium dioxide layer coats a graphene-silicon solar cell. The cell appears blue, because the coating has reduced visible light reflection from the silicon surface (Courtesy of Anyuan Cao, Peking University)
Researchers from Peking University discovered that graphene-silicon solar cells can become more efficient by coating them with an anti-reflective layer such as Titanium Dioxide. The researchers say that normal silicon solar cells have an efficiency of about 15%. Adding graphene (as an electrode and also as the charge-carrying layer) can make the cells cheaper, but these are less efficient (at 8.6%). Coating these structures with a 65-nm-thick layer of titanium dioxide decreases the reflected light from over 30% to less than 10%. This means that more light is converted to electricity yielding an efficiency of 14.6% for such cells.