Lattice Light Sheet Microscopy (LLSM) is a variant of conventional Light Sheet Microscopy (LSM). In short, LLSM utilizes a lattice of light beams to image a specimen. While LSM techniques illuminate single planes at a time, LLSM scans the whole specimen. It does this by a clever choice of instruments and borrowed principles from Bessel beam microscopy and structured illumination microscopy (SIM). The best thing about LLSM is its aid in live, unperturbed imaging of systems. It also has low phototoxicity to samples compared to confocal microscopy and 2-photon microscopes.
In 2010, Eric Betzig developed LLSM in hopes that it would aid in imaging areas current microscopes could not capture. Today, Lattice Light Sheet Microscopy is still in its early age. However, it is emerging as a top choice for imaging living systems. Along with its gentleness in preventing photodamage, it also boasts a high data acquisition rate. LLSM can image from 200 to 1000 planes per second. This is a whole order of magnitude faster than traditional Bessel beam and confocal microscopes. Clearly, it can produce much more continuous videos than was possible before its time.
Lattice Light Sheet Microscopy also finds usage in detecting growth and development of living cells, specifically, the embryonic cells. Embryonic systems are notorious for being difficult to capture with conventional fluorescent microscopes. This is due to their delicate nature and the speed at which these systems move. On the other hand, LLSM can capture subcellular processes of embryos without damaging tissue. In this article, we will discuss the workings of LLSM and how it applies to live embryonic imaging.
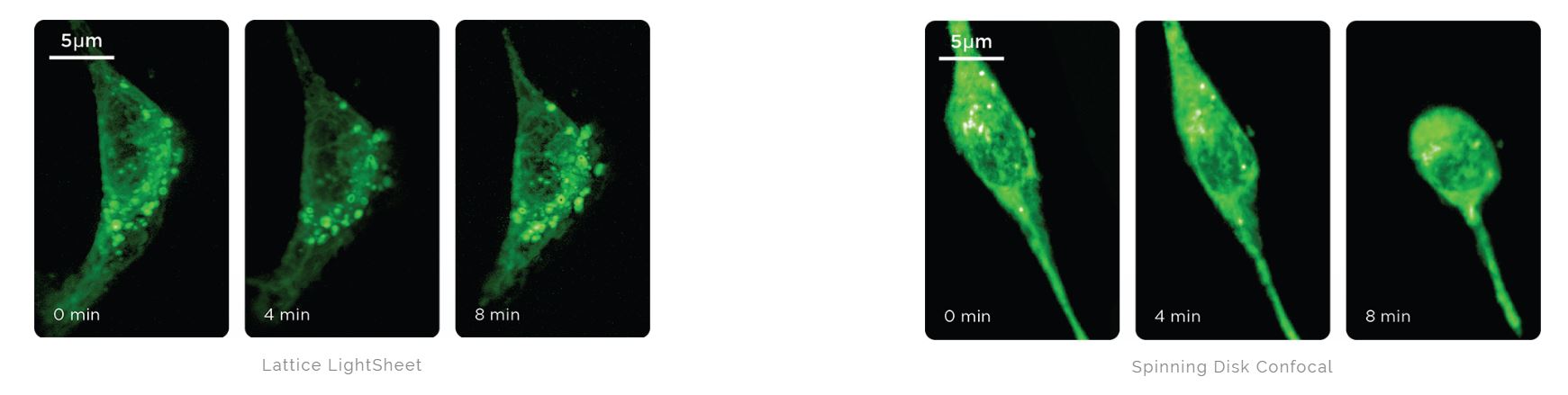
Mouse embryonic fibroblasts expressing SiR-Actin (642 nm) imaged with 25 ms exposures. After 8 minutes, the cells died on the spinning disk confocal but the Lattice Light Sheet cells remained viable. Courtesy of 3i.
How Light Sheet Microscopy Works
Imaging with Lattice Light Sheet Microscopy works through a series of steps. First, the lattice sheet must be setup with several instruments such as two pairs of cylindrical lenses, a light modulator, and an optical mask. The lattice of light sheets starts with the input laser beam (typically, this beam is circular and linearly polarized).
Each pair of cylindrical lenses is used to create a sheet of light from the input beam. Each lens is setup to help stretch the beams towards two different perpendicular axes. Conventionally, this will be the x and z axes. Now, the modulator will take these sheets and take away any excess diffraction. This stage is where a preliminary lattice of “Bessel beams” is formed. Lastly, an opaque optical mask is used to double check the lattice for extra diffraction. It will also lengthen the light so that the image will be projected into the z-plane.
An array of excitation lasers is used on the lattice light sheet microscope for illuminating the specimen. Courtesy of Janelia Research.
Now, the lattice can help take images or live videos of the specimen. An electron multiplying charge coupled device (EMCCD) camera is highly recommended. Each lateral plane of the specimen is taken into study and put together into a 3D composite image. For photos, the sample is scanned over once and for videos, the sample will be scanned continuously. An electron multiplying charge coupled device (EMCCD) camera is highly recommended to capture these.
LLSM Imaging Modes
An LLSM device can be further modified to provide different imaging modes. We will discuss three of these modes. First, the sheet scan is when the lattice and objectives are moved in unison to create 3D images.
Next is the sample scan mode. Here, the instruments remain stationary, but the sample is moved. This method is convenient for larger specimens where moving the objectives would require larger platforms.
Lastly a structured illumination mode (SIM) helps create a more clear image. The clarity is beyond what the diffraction limits of the objectives would normally allow. Apart from this, SIM requires several rotations of the light sheet on the stationary sample. At each angle, an additional grid pattern of light is superimposed onto the sample. This grid will help the computer algorithms calculate and create composite images from all the steps.
Embryo Imaging
LLSM has been utilized in studies on tracking single molecule development in fruit fly and mouse embryonic development. Specifically, Mir et al. conducted a study focused on tracking single molecules of the transcription factor Bicoid. They were able to track nuclear divisions and spatial positions of elements in these embryos. Additionally, they found a series of optical adjustments that optimized their imaging technique for this fly species. Some of the important points of these adjustments include choosing the right exposures and good embryo positioning.
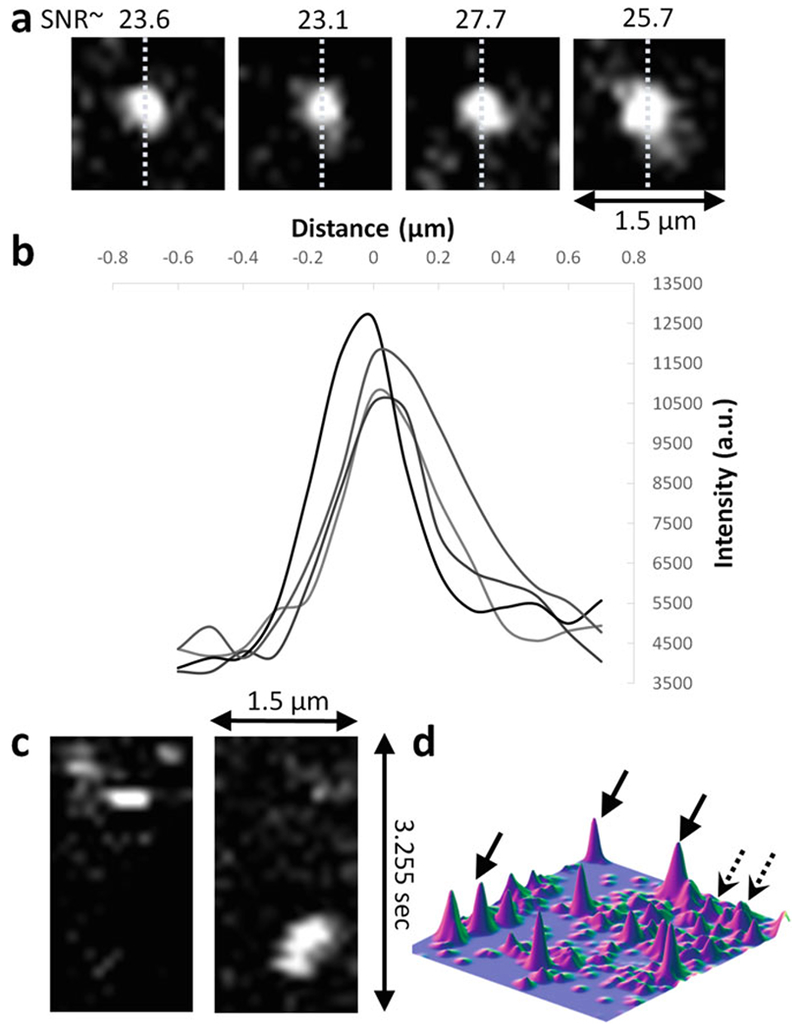
Examples of verification of ability to detect single molecules on data acquired at 100 ms exposure times on Bicoid-GFP. (a) Single molecule detections. Dashed lines indicate the lines used to plot the profiles shown in (d). (b) Line profiles corresponding to the example detections shown in (a). (c) x–t slice of a single molecule detections exhibiting single step loss of signal. (d) Surface plot of an image with several single molecule detections. Dashed arrows are from out-of-focus or rapidly moving molecules. Courtesy of Mir et al.
More Tips
Within this experiment, they marked all the x, y positions of the embryos and found ones at the wanted developmental stage. Another important tip they found was to make the surface of the embryo as close to the equipment as possible. This would make it possible to minimize exposure to excitation and emission beams. The research team also projects that doing future studies on proteins, RNA, and DNA will help gain understanding of these species’ development.
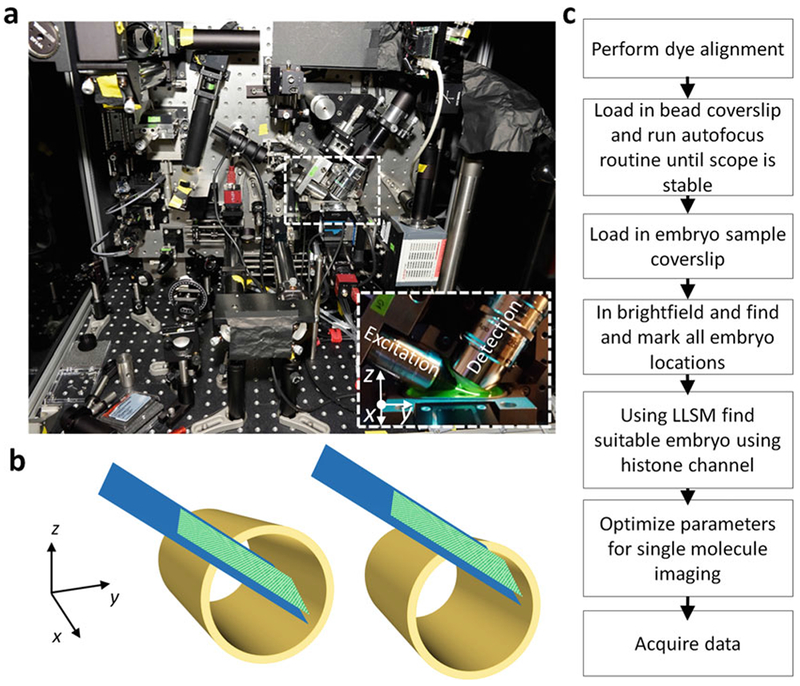
Coordinate system of the sample navigation stages with respect to the detection and excitation objectives. (a) Picture of the lattice light-sheet microscope with zoomed in image of sample chamber, excitation and detection objectives. (b) How to position the embryo within the excitation sheet: hollow cylinder- embryo, solid plane- excitation illumination (c)Workflow for LLSM alignment and data aquisition. Courtesy of Mir et al.
Conclusion
Lattice Light Sheet Microscopy is a clear alternative to traditional fluorescent microscopy for sensitive systems. As we have discussed, LLSM can take high frame rates and output high-resolution data. Moreover, LLSM boasts adjustable imaging modes and parameters. It is even possible to take images with multiple colors, and videos for hours on end. The good characteristics do not stop there: because of LLSM’s versatility, it may be possible to learn more about immunology. Studies have already started on T-Cell receptors. More than ever, we have a need for these techniques and studies in our globalized world.
This post was sponsored by Gentec Electro-Optics, Inc. - world leader in laser beam measurement solutions
