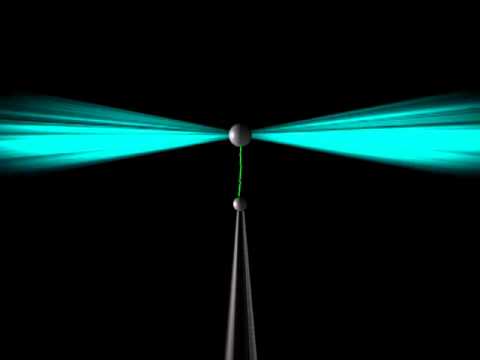
Optical tweezers can trap single living cells for many analytical purposes. Courtesy of Youtube.
Researchers at Heriot-Watt University in Edinburgh, UK have developed a new design for optical tweezers using a single diode-coupled multicore fiber. The novel method promises a much more flexible and accessible setup to analyze single cells for a better understanding of many biological systems.
Optical tweezers utilize light to essentially “trap” microscopic objects. Highly-focused laser beams project on to micron-sized dielectric particles. The particles are attracted to the very strong electric field gradient at the beam waist. This creates a small force at the center, effectively holding the particles in place and allowing for manipulation. Trapping living cells allows researchers to analyze cell structure and intercellular interactions.
Georgia Anastasiadi, Mark Leonard, Lynn Paterson, and William N. Macpherson describe their approach to optical tweezers in the February, 2018 issue of Optics Express. The team writes that their single fiber model provides a final quality factor that is “in line with conventional optical tweezers, but allowing more flexibility in the type of trapping and imaging experiments that can be performed.”
History of Optical Tweezers
According to the paper, traditional optical tweezers setups suffer from limits of directing a trapping beam through a high numerical aperture (NA) objective lens. While this allows for a tightly focused beam, the high NA lens often causes spherical aberration of the beam, a limited field of view, or limited trapping force strength. Others have proposed fiber-based systems to remedy this in the past. These methods, however, typically use two or more opposed fibers to trap particles along the optical path. Multiple fiber based systems require very precise fiber alignment and struggle to render full 3D manipulation over the particle. The team set out to address the potential shortcomings of a multiple fiber system while eliminating restrictions caused by high NA objective lenses.
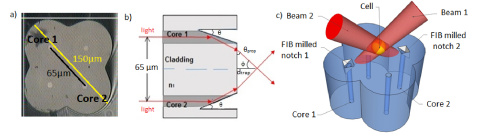
The above drawings give a look at the inside of the single multicore fiber and installed mirrors. The figure on the right shows projections of the trapping beams. Courtesy of OSA publishing.
The Design
This new diode-driven process involves a single multicore fiber. Two laser diodes both emit a 975nm beam into separate diagonally opposite cores so that the power within each can be changed independently of one another. Two mirrors are cut into these cores at an angle that causes total internal reflection. The beams will therefore propagate through the core, strike a mirror, then direct toward the center of the fiber where they will cross. Thus, a potential trap forms.
The researchers greatly reduce the distance between active fiber cores by using a single multicore fiber instead of the four fiber bundle used earlier. By their estimate, this distance is reduced by about half in their experimental setup. The single fiber method also eliminates the need for tedious and precarious fiber gluing.
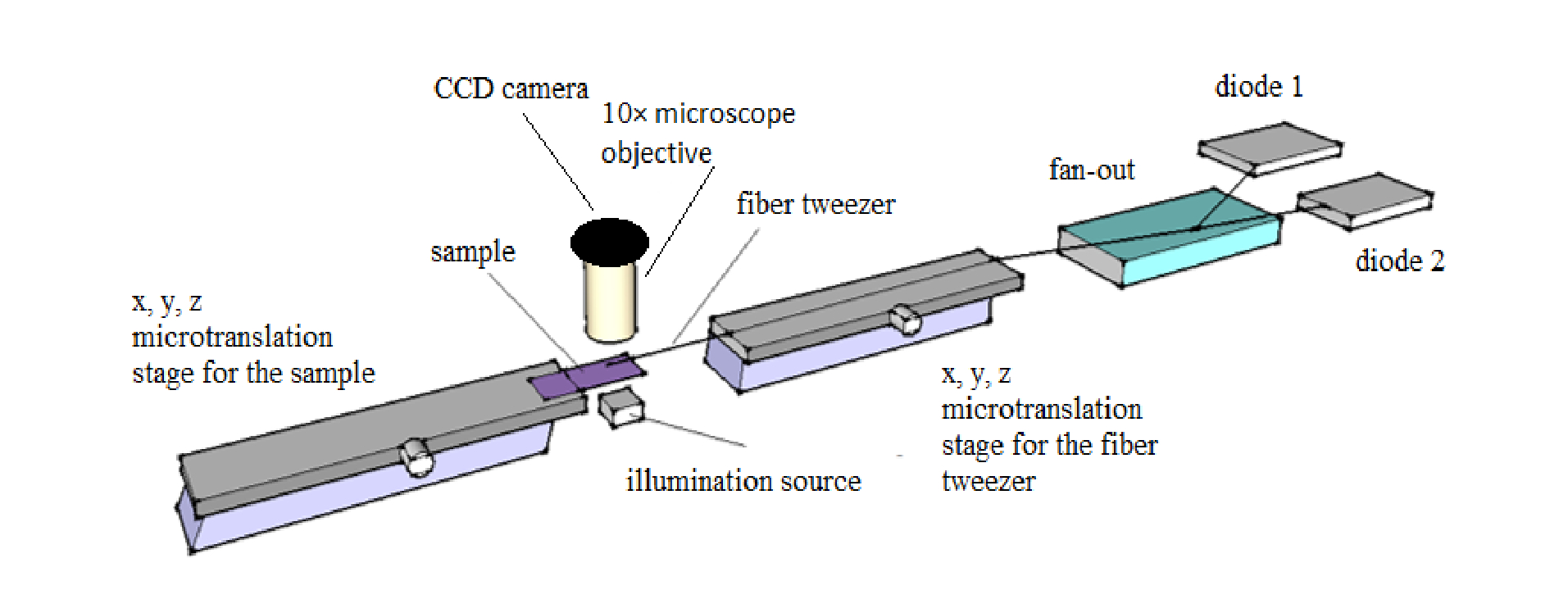
The above shows the experimental setup of the single fiber optical tweezers. Courtesy of OSA publishing.
The research team’s complete system consists of the diode-coupled fiber, two translation stages for adjustments in the x,y, and z directions, a slide for the sample, and a microscope objective and CCD for imaging. One translation stage works to align the fiber tweezer while the other shifts the sample. Here, the team chose to trap yeast cells submerged in water at room temperature.
The Results
The cells were successfully trapped and manually moved along all three dimensions. The team was able to hold single cells in place without needing to modify the microscope system because their design decouples the fiber from the imaging system. That is, the trapping ability is no longer limited by the objective lens. This has especially important implications in Raman spectroscopy. Single cell Raman spectroscopy is notoriously difficult because fluid flow in the sample typically causes cell movement. The Raman signal is also dominated by the background signal of the microscopic slide. Researchers successfully demonstrated how their design remedies these concerns by manipulating the cell into a stable position above the slide.
The simplified single fiber setup resolves alignment and available space concerns without compromising overall trapping ability. This portable and flexible optical tweezers system promises to be a great advancement in non-invasive single cell experimentation. For additional information on the design process and implementation of the fiber tweezers by Anastasiadi, Leonard, Paterson, and Macpherson, please see their study below.
https://doi.org/10.1364/OE.26.003557
Optics Expres Vol 26, Issue 3, p. 357-3567 (2018)
