Vision loss from vitreoretinal diseases affects millions worldwide, presenting a significant health challenge. Enter optogenetics—a cutting-edge approach that harnesses the power of light to manipulate cells within the eye. This article delves into how optogenetics could revolutionize treatment paradigms, offering new hope to those at risk of losing their sight. Read on to discover the science and potential of this promising therapy.

Vitreoretinal disease is a form of eye disease which affects the retina and surrounding vitreous fluid. Approximately 12 million Americans have some form of vitreoretinal disease, which can cause vision loss. Optogenetics is a new treatment therapy being explored, with promising results. Image courtesy of CDC.
1. Introduction to Vitreoretinal Diseases
Vitreoretinal diseases encompass a range of conditions that affect the retina and the vitreous humor, the clear gel-like substance filling the eye between the lens and the retina. These disorders impact the retina, a crucial, light-sensitive layer of tissue at the back of the eye, responsible for capturing images and sending visual information to the brain via the optic nerve.
Damage to the retina can result from various causes, with diabetes and aging being predominant factors. The most prevalent vitreoretinal conditions include Wet Age-Related Macular Degeneration (Wet AMD), Diabetic Retinopathy, Diabetic Macular Edema, and Retinal Vein Occlusion (RVO). According to the National Eye Institute, approximately 7.7 million Americans are affected by diabetic retinopathy alone, with projections suggesting this number could double by 2050. Similarly, age-related macular degeneration is the leading cause of vision loss among people aged 50 and older, affecting over 11 million individuals in the United States.
Given the significant prevalence and the severe impact of these diseases on vision, substantial research efforts are dedicated to finding effective treatments. One innovative approach currently being explored is optogenetics, which offers potential for precise, controlled therapeutic intervention at the cellular level in the retina.
2. Understanding Optogenetics: Controlling Cellular Activity with Light
Optogenetics is a transformative field that leverages light and laser technology to control and modify cellular activities in living organisms. This technology primarily manipulates cellular behavior through light-gated ion channels. By genetically modifying cells to express photosensitive proteins within their cell membranes—the lipid bi-layers that enclose and protect cells—scientists can precisely control these cells. When targeted cells are exposed to light at specific wavelengths, the engineered ion channels within the cell membranes open, allowing ions to flow across the membrane. This ion movement shifts the membrane potential significantly, triggering an action potential that transmits information along neurons throughout the body.
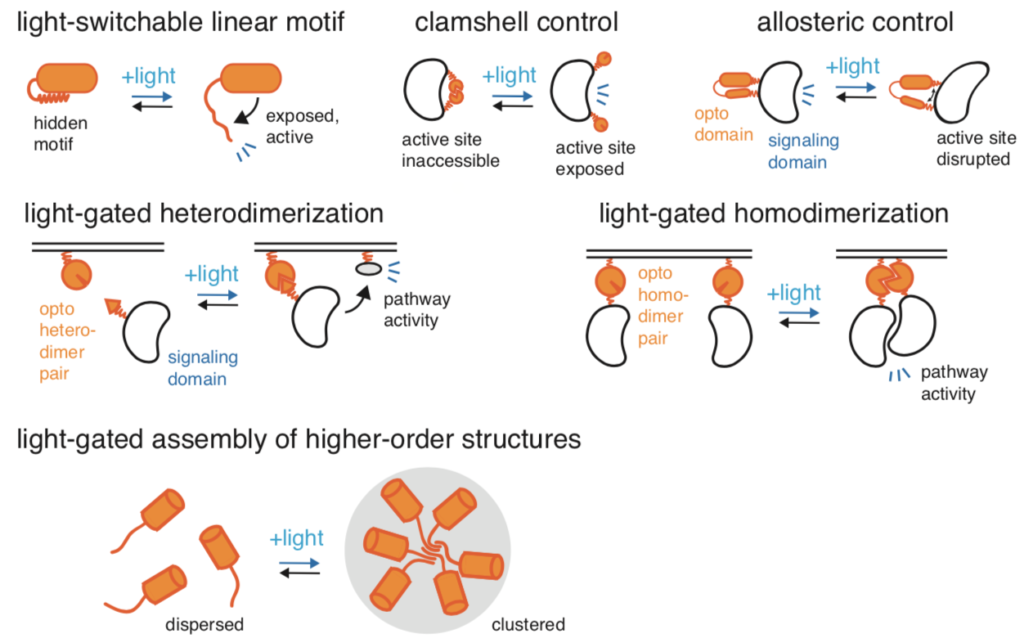
This image details various ways in which applied light modifies proteins within cells. As shown, light has more applications than just the modification of light-sensitive ion channels, although that is the main application of optogenetics. Image courtesy of Current Opinion in Chemical Biology.
The most commonly used proteins in optogenetic studies are channelrhodopsin, halorhodopsin, and archaerhodopsin. These proteins are responsive to light wavelengths of approximately 473nm, 660nm, and 561nm, respectively. These wavelengths fall within the visible light spectrum, making diode lasers and femtosecond lasers the preferred tools for activating these channels due to their precision and control.
This process not only allows for the detailed study of cellular responses and neural pathways but also opens new avenues for medical research, where cellular functions can be controlled with unprecedented precision. The accompanying image further illustrates the diverse ways light can be used to control cellular and protein activity, highlighting the versatility and potential of optogenetics in research and therapy.
3. The Science Behind Optogenetics
In this section, we provide a detailed explanation of the underlying principles of optogenetics. We discuss its mechanism at the cellular level, the roles of light-sensitive ion channels, and the essential tools and techniques employed in optogenetic research.
3.1. Understanding the Cellular Mechanics
Optogenetics is a revolutionary technique that integrates genetics and optics to control and monitor the activities of individual neurons in living tissue using light. At its core, optogenetics involves the insertion of genes encoding light-sensitive proteins into specific types of cells. Once expressed, these proteins alter the electrical properties of the cells’ membranes when illuminated by light of certain wavelengths. This light-induced change in voltage across the membrane can activate or inhibit the neuron, mimicking or blocking natural electrical signals within the brain.
3.2. Roles of Light-Sensitive Ion Channels
The proteins used in optogenetics, known as opsins, are primarily ion channels or pumps sourced from various organisms, such as algae and bacteria. Channelrhodopsin (ChR2), for example, allows positive ions to enter the cell upon activation by blue light, leading to cell depolarization and activation. Conversely, halorhodopsin (NpHR) responds to yellow light to pump chloride ions into the neuron, hyperpolarizing it and effectively inhibiting its activity. This ability to selectively control neurons either to fire or to remain silent underlies much of the power of optogenetic methodologies in dissecting neural circuits.
3.3. Tools and Methods in Optogenetic Research
The application of optogenetics requires a set of sophisticated tools and techniques:
- Genetic Engineering: Techniques such as viral vectors are used to deliver opsin genes specifically to targeted cells. This precision is obtained either by employing promoters that activate exclusively in certain cell types or through approaches like CRISPR for accurate gene editing. The term “CRISPR,” pronounced “crisper,” stands for Clustered Regularly Interspaced Short Palindromic Repeats. These sequences are critical components of a bacterial defense system, which forms the foundation of the CRISPR-Cas9 genome editing technology.
- Optical Devices: Once the opsins are expressed, researchers use specially designed optical devices to deliver light directly to the cells of interest. These devices range from fiber-optic cables that deliver light to deep brain structures to more complex systems that allow for the simultaneous illumination of different brain areas with different light colors and patterns.
- Imaging and Recording Technologies: To monitor the effects of optogenetic manipulation, researchers often use advanced imaging techniques like calcium imaging, which can visualize neuronal firing in real-time. Electrophysiological recordings are also used to measure the precise changes in neural activity induced by light stimulation.
By integrating these tools and methods, optogenetics allows for an unprecedented level of control and observation of neural circuits, offering profound insights into the fundamental workings of the brain and paving the way for potential new treatments for neurological and psychiatric disorders.
4. Application of Optogenetics in Treating Vitreoretinal Diseases
In this treatment form, light-sensitive proteins are inserted into retinal neurons which did not initially have a light sensitivity. This allows for phototransduction in the retina to occur when original photoreceptors are damaged. Depending on the location or extent of the damage, different cells are capable of modification. For example, with significant retinal damage, retinal ganglion cells are targeted for modification with light-sensitive proteins. Viral vectors are most commonly used for protein delivery. For ophthalmology, the rhodopsin family of proteins is used; these proteins covalently bind the chromophore retinaldehyde (RAL). The RAL bonded to type 1 rhodopsins undergoes a conformational change after exposure to light, which allows for the opening of an ion channel. Channelrhodopsin and halorhodopsin belong to the type 1 rhodopsin group. Type 2 opsins undergo intracellular G-protein–coupled signaling cascades after light exposure.
This image displays the three primary retinal cells targeted for optogenetic treatment. The figure to the left displays their location and relation to one another. The figures on the right displays the opsins used as well as membrane potential over time after being exposed to light. Image courtesy of Ophthalmic Communications Society, Inc.
When signaling channels from the retinal cells to the brain along neurons are restored, vision can be partially or fully restored. Current research trials are investigating improved amplification and signaling farther down the signaling pathway.
One main problem with optogenetic treatment is that type-1 opsins require high levels of light to promote activity. These high levels of light can be achieved with an external light source, such as a femtosecond laser. However, this could cause damage in terms of photo-toxicity. Future research can be done to minimize harmful effects of external light sources, or to minimize necessary light required by the opsins.
5. Case Studies and Clinical Trials
In this section we look into two pivotal studies and clinical trials that have explored the application of optogenetics in treating eye diseases, particularly those affecting the retina. We summarize the significant findings and outcomes, highlighting advancements in the field:
5.1. Key Studies Overview:
Study by Jens Duebel et al., 2015: This study discusses the development of novel opsins with enhanced light sensitivity and their potential use in the retina. It outlines how microbial opsins, improved through red-shifted action spectra, allow for safe optogenetic stimulation below the human eye’s safety threshold.
- Objective: This study aimed to explore the potential applications of optogenetics in ophthalmology, particularly for restoring vision in patients with retinal degenerative diseases.
- Methodology: Researchers focused on developing microbial opsins with enhanced light sensitivity. These opsins were genetically engineered to respond to light levels that are safe for human eyes.
- Findings: The study introduced opsins with red-shifted action spectra, which are capable of activating under lower light levels than previously possible. This advancement not only increases the safety of optogenetic treatments but also makes them more viable for clinical applications. The opsins developed show potential in restoring partial vision by making light-sensitive proteins available to retinal cells that have lost their natural photoreceptor functions.
- Impact: This research paves the way for using optogenetics as a non-invasive alternative to traditional treatments for eye diseases like age-related macular degeneration and retinitis pigmentosa.
Advancements in Viral Vectors: The research emphasizes the success in creating highly efficient viral vectors for ocular gene therapy, marking a crucial step toward practical optogenetic applications for vision restoration.`
- Objective: To enhance the delivery mechanisms of optogenetic tools into the retina, thereby increasing the efficiency and specificity of gene therapy treatments.
- Methodology: The research focused on optimizing adeno-associated viral (AAV) vectors, which are commonly used in ocular gene therapy due to their safety profile and ability to target various retinal cell types.
- Findings: Significant progress was made in developing highly efficient viral vectors that can effectively deliver genetic material to the retina. These vectors have been engineered to achieve better penetration and more precise targeting, reducing potential side effects and increasing the effectiveness of the gene delivery.
- Impact: The advancements in viral vector technology are crucial for the practical application of optogenetics in vision restoration. Enhanced vectors facilitate the stable introduction of light-sensitive proteins into targeted retinal cells, potentially allowing for the widespread adoption of optogenetic therapies in clinical settings.
These key studies underscore the dynamic nature of optogenetic research and its trajectory towards becoming a transformative tool in treating vitreoretinal diseases, offering hope for improved quality of life for patients suffering from vision impairment.
5.2. Tangible Advancements:
Let’s look into into the tangible advancements achieved through the application of optogenetic therapies in vision restoration. We examine the efficacy of introducing light-sensitive proteins into retinal cells and the innovative strides made in vector technology, highlighting their combined potential to enhance treatment outcomes for eye diseases without the need for invasive procedures.
Restoration of Light Sensitivity:
- Effectiveness: The genetic introduction of light-sensitive proteins into retinal cells has demonstrated significant success in restoring light sensitivity to cells that had lost their natural photoreceptive capabilities. This approach is especially promising for patients with conditions like retinitis pigmentosa or advanced macular degeneration, where photoreceptors degrade over time.
- Procedure: The process involves using optogenetic techniques to modify specific types of retinal cells, enabling them to respond to light stimuli. This restoration of light sensitivity is achieved without the need for invasive surgical interventions, offering a less risky alternative to traditional eye surgeries.
Advancements in Gene Delivery:
- Innovations: Recent innovations in vector technology, particularly the development and refinement of adeno-associated viral (AAV) vectors, have significantly improved the precision and efficiency of gene delivery to the retina.
- Impact: These advancements have enabled more targeted delivery of therapeutic genes to the desired types of retinal cells. The ability to precisely target these cells enhances the efficacy of the treatment, ensuring that the optogenetic modifications achieve the desired therapeutic effects with minimal off-target impacts.
- Clinical Relevance: The improved delivery methods have not only enhanced the success rates of experimental treatments but also have raised the potential for these techniques to be used in broader clinical applications. This is a critical step toward making optogenetic therapy a viable option for routine clinical use in ophthalmology.
These outcomes highlight the progress being made in the field of optogenetics, illustrating its potential to revolutionize the treatment of retinal diseases by providing non-invasive, targeted, and effective treatment options.
6. Other Potential Treatments
Exploring treatments beyond optogenetics offers a comprehensive view of the evolving landscape for managing vitreoretinal diseases. Notable alternatives include stem cell therapy, gene-based therapies, and retinal prosthetics, each with its own set of advantages and challenges.
Stem Cell Therapy: Human pluripotent stem cells hold the potential to generate all necessary cells for retinal regeneration. This approach promises substantial benefits, but transplantation carries risks, including the formation of teratomas (tumors arising from stem cells). Despite the potential, clinical applications require stringent safety protocols to mitigate these risks.
Gene-Based Therapies: Gene therapy involves altering the genetic makeup of retinal cells to counteract damage or confer protection against future harm. While this method shows significant promise, unintended genetic mutations can occur, leading to unforeseen consequences. Additionally, gene therapy often sparks ethical debates, emphasizing the need for careful consideration and regulation.
Retinal Prosthetics: Retinal implants, or prosthetics, have demonstrated success in restoring some degree of vision. These devices must be customized to match the specific damage of an individual’s retina, and currently, they offer limited resolution. However, ongoing advancements in technology may improve their efficacy and accessibility.
While these treatments represent significant strides in combating vitreoretinal diseases, each presents unique challenges and risks that must be addressed through continued research and clinical trials. The evolving field of optogenetics, in conjunction with these therapies, underscores the dynamic and multifaceted approach needed to restore vision and improve quality of life for patients with retinal diseases.
For a detailed exploration of optogenetics and other potential treatments for vitreoretinal diseases, you can read the full paper here.
7. Future Directions and Research
The future of optogenetics holds immense potential, driven by emerging trends and ongoing research efforts aimed at expanding its medical applications. As the field progresses, several key areas warrant attention.
Emerging Trends in Optogenetic Research: Recent advancements in the development of opsins with enhanced light sensitivity and red-shifted action spectra have paved the way for safer and more efficient optogenetic therapies. Studies have demonstrated that microbial opsins can now be activated at light levels well below the safety threshold for the human eye, reducing the risk of phototoxicity. Additionally, innovations in viral vector technology have significantly improved the delivery of optogenetic tools to targeted retinal cells, enhancing treatment efficacy and precision.
Potential New Applications in Medical Treatment: Beyond ophthalmology, optogenetics shows promise in treating a range of neurological and psychiatric disorders. For instance, research is exploring the use of optogenetics to modulate neural circuits involved in conditions such as Parkinson’s disease, epilepsy, and depression. Preliminary studies have indicated that optogenetic interventions can restore normal neural activity and alleviate symptoms in animal models, suggesting potential for future clinical applications.
Challenges and Ethical Considerations: Despite its promise, optogenetics faces several challenges that must be addressed to ensure its safe and effective implementation. One major hurdle is the potential for immune responses against microbial opsins when introduced into human tissues. Researchers are actively working on engineering opsins with reduced immunogenicity to mitigate this risk. Additionally, ethical considerations surrounding gene editing and the long-term effects of optogenetic therapies must be carefully evaluated. The potential for unintended off-target effects and the need for precise control over gene expression are critical areas of concern that require ongoing research and robust regulatory frameworks.
If you found this article informative, you might also be interested in "DNA Sequencing: Photonics in Genome Analysis".
8. Conclusion
Optogenetics represents a transformative approach to treating vitreoretinal diseases, offering hope for restoring vision where traditional methods have fallen short. By utilizing light-sensitive proteins to activate retinal cells, this innovative therapy can potentially reverse the effects of conditions like retinitis pigmentosa and age-related macular degeneration. The advancements in opsin development and viral vector technology have brought us closer to safe and effective clinical applications, highlighting the significant strides made in recent years.
Despite these promising developments, continued research is essential to address the remaining challenges and refine these treatments. The potential for immune responses, ethical considerations, and the need for precise gene control underscore the importance of ongoing studies and collaborations.
We encourage the scientific community and the public to support and engage in further research and awareness initiatives. By fostering a collaborative environment and maintaining a focus on innovation and ethical responsibility, we can unlock the full potential of optogenetics to improve the quality of life for those affected by vitreoretinal diseases.
9. References
- Duebel, J., Marazova, K., & Sahel, J.-A. (2015). Optogenetics. Current Opinion in Ophthalmology, 26(3), 226-232. doi:10.1097/ICU.0000000000000140.
- Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G., & Deisseroth, K. (2005). Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neuroscience, 8(9), 1263-1268.
- Cronin, T., Vandenberghe, L. H., Hantz, P., et al. (2014). Efficient transduction and optogenetic stimulation of retinal bipolar cells by a synthetic adeno-associated virus capsid and promoter. EMBO Molecular Medicine, 6(9), 1175-1190.
- Busskamp, V., Duebel, J., Balya, D., et al. (2010). Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science, 329(5990), 413-417.
- Mace, E., Caplette, R., Marre, O., et al. (2015). Targeting Channelrhodopsin-2 to ON-bipolar cells with vitreally administered AAV restores ON and OFF visual responses in blind mice. Molecular Therapy, 23(1), 7-16.
- Sahel, J.-A., Roska, B. (2013). Gene therapy for blindness. Annual Review of Neuroscience, 36, 467-488.
- Dalkara, D., Byrne, L. C., Klimczak, R. R., et al. (2013). In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Science Translational Medicine, 5(189), 189ra76.
- Hauswirth, W. W., Aleman, T. S., Kaushal, S., et al. (2008). Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Human Gene Therapy, 20(9), 999-1004.
- Maguire, A. M., Simonelli, F., Pierce, E. A., et al. (2008). Safety and efficacy of gene transfer for Leber’s congenital amaurosis. New England Journal of Medicine, 358(21), 2240-2248.
- Klapoetke, N. C., Murata, Y., Kim, S. S., et al. (2014). Independent optical excitation of distinct neural populations. Nature Methods, 11(3), 338-346.
These references provide the scientific foundation and additional reading for the discussed advancements and clinical trials in optogenetics and other potential treatments for vitreoretinal diseases.

Excellent articles about the vitreoretinal disease, optogenetic therapy, and other treatments.